40 fda approved health claims on food labels
FDA Takes Steps to Allow Qualified Health Claims on Labels By Keller and Heckman LLP's Packaging Practice Group. The Food and Drug Administration announced March 31, 2004, that a qualified health claim will soon appear on product labels for walnuts and the reduced risk of coronary heart disease. "This qualified health claim is part of the FDA's initiative to provide Americans with better information ... Use of the Term Healthy on Food Labeling | FDA The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance. Updating the "healthy"...
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA ...

Fda approved health claims on food labels
The FDA Wants to Update the Definition for "Healthy" Claims on Food Labels A new Food and Drug Administration proposed rule, "Food Labeling: Nutrient Content Claims; Definition of Term Healthy," released on September 28, would offer new guidance to brands who label their products as "healthy" options. The FDA is looking to regulate the use of the word because of the rise of diet-related illnesses in the U.S. Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables)... Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim"
Fda approved health claims on food labels. FDA perspectives on health claims for food labels - PubMed FDA perspectives on health claims for food labels Authors J Craig Rowlands 1 , James E Hoadley Affiliation 1 Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, College Park, MD 20740, USA. JCRowlands@Dow.com PMID: 16480811 DOI: 10.1016/j.tox.2005.10.023 Food Labeling* Legislation, Food* Nutritive Value Research Design Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food... Is It Really 'FDA Approved'? | FDA - U.S. Food and Drug ... May 10, 2022 · The FDA is responsible for protecting public health by regulating human drugs and biological products, animal drugs, medical devices, tobacco products, food (including animal food), cosmetics, and ... Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims;...
FDA Drug Safety Communication: FDA cautions about using ... [03-03-2015] The U.S. Food and Drug Administration (FDA) cautions that prescription testosterone products are approved only for men who have low testosterone levels caused by certain medical ... Food and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ... Daily Value on the New Nutrition and Supplement Facts Labels Feb 25, 2022 · However, they are required to list any vitamins and minerals that are added to the food or if a statement is made on the package labeling about their health effects or the amount contained in the ... Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the...
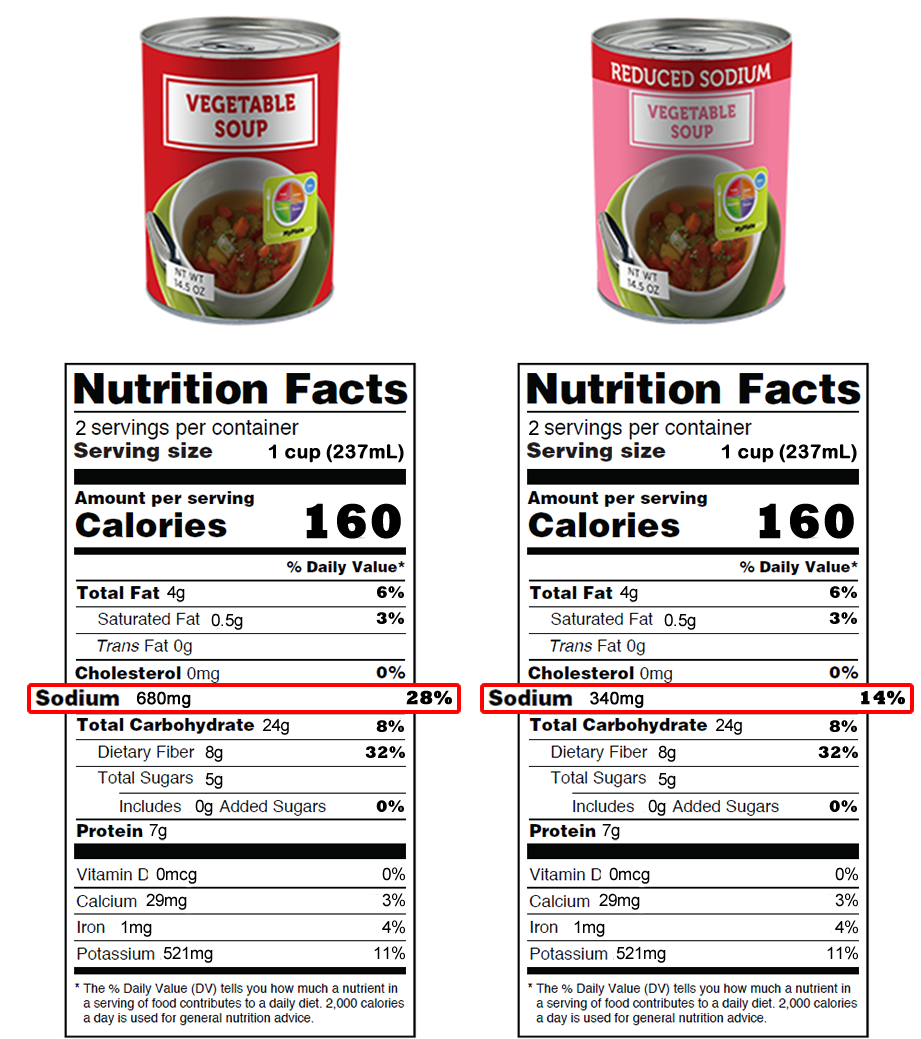
Qualified Health Claims | FDA - U.S. Food and Drug Administration The process does not involve rulemaking. For more information, visit Questions and Answers: Qualified Health Claims in Food Labeling or explore the linked pages below. Qualified Health... FDA approves cardiovascular health claim on certain oil labels ... The FDA-approved health claim is as follows: "supportive but not conclusive scientific evidence suggests that daily consumption of about 1½ tablespoons (20 grams) of oils containing high... Approved Fda Health Claims FDA Announces Qualified Health Claim for Magnesium and Reduc… Health (8 days ago) People also askWhat does FDA approved and CLIA waived mean?What does FDA approved and CLIA waived mean?Clia FDA approved Clinical Laboratory Improvement Amendments, i.e., CLIA waived tests establishes quality standards for all laboratory testing to ensure the accuracy, reliability. Everything you need to know about Health Claims on Food Labels The FDA has approved 12 health claims on food labels such as sodium and hypertension; fiber-containing grains, fruits and vegetables and cancer; and more. Qualified health claims are supported by some scientific evidence and reviewed by the FDA, but do not meet the significant scientific agreement standard.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient...
Health claims on food labels - Food labels - Canadian Food ... A health claim is any representation in labelling or advertising that states, suggests, or implies that a relationship exists between the consumption of a food and health. All aspects of food labels and advertisements contribute to the overall impression made by a food product, including health claims.
Introduction to Food Product Claims — FDA Reader There are two types of health claims that appear on food labels and marketing. They are: Authorized Health Claims Qualified Health Claims Requirements for a Health Claim Health claims cannot be made about the diagnosis, cure, mitigation or treatment of diseases (this is a drug claim) They must be complete, truthful and not misleading.
Solved: What FDA-approved health claims are used on food package l ... Health claims are statements which are made by the manufactures and approved by the law. Food and Drug Administration (FDA) has laid down health claims to be used on the food package, specifying which nutrient present in the food has a potential of reducing risk of a disease or health condition.
Making Health Claims on Food Labels: The FDA Rules You Need to Know If you want to make health claims on your food labels, it's important to understand the FDA's guidelines and how to obtain accurate nutrition labels. ... Making Health Claims on Food Labels: The FDA Rules You Need to Know. By lcadmin. Posted March 12, 2018. In FDA regulations, Food labeling
How to Start a Food Business | FDA - U.S. Food and Drug ... Food Businesses Subject to FDA Regulation. FDA regulates all foods and food ingredients introduced into or offered for sale in interstate commerce, with the exception of meat, poultry, and certain ...
FDA proposes updates to 'healthy' claim on food packages | CNN In order to be labeled with the "healthy" claim, products would need to: Contain a certain, meaningful amount of food from at least one of the food groups or subgroups - such as fruits,...
What You Need to Know About Health Claims on Food Labels and Dietary ... In general, health claims are statements made on food product labels or dietary supplements that boast some type of health benefit. This may seem simple, but the FDA doesn't treat every claim the same way. Label claims come in multiple forms: Health claims (which comprise of authorized health claims and qualified health claims)
Food Packaging Claims | American Heart Association There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims. A "health claim" by definition has two essential components: A substance (whether a food, food component, or dietary ingredient) and
FDA Proposes to Update Definition for "Healthy" Claim on Food Labels The "healthy" claim can act as a quick signal on food package labels to help empower consumers, including those with lower nutrition knowledge, with information to identify foods that will...
FDA Proposes New 'Healthy' Claim on Food Labels FDA Proposes New 'Healthy' Claim on Food Labels. Sept. 28, 2022. Its food group-based approach continues prohibitions but allows salmon and nuts to be considered healthy. The FDA today (Sept. 28) issued a proposed rule to update the definition of the "healthy" claim on food & beverage packaging. Interested parties have about three ...
Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are...
Nutrition 1020 - Module 2 Assessment Flashcards | Quizlet Study with Quizlet and memorize flashcards containing terms like Which of the following is a potentially biologically harmful substance that is required by law to be identified on a food package?, The term free can be used in reference to:, Which health condition does not have a FDA-approved health claim? and more.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). Sec. 101.14 Health claims: general requirements. (a) Definitions. For purposes of this section, the following definitions apply: (1) Health claim means any claim made on the label or in labeling of a food, including a dietary supplement ...
The FDA Wants to Update the Definition for "Healthy" Claims on Food Labels Danielle DeAngelis. A new Food and Drug Administration proposed rule, "Food Labeling: Nutrient Content Claims; Definition of Term Healthy," released on September 28, would offer new guidance to brands who label their products as "healthy" options. The FDA is looking to regulate the use of the word because of the rise of diet-related illnesses ...
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
Structure/Function Claims | FDA - U.S. Food and Drug ... Mar 07, 2022 · Final Rule: Food Labeling: Nutrient Content Claims, Health Claims, and Statements of Nutritional Support for Dietary Supplements (62 Fed. Reg. 49859 at 49863-49866) Conventional Foods
Qualified Health Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim"
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables)...
The FDA Wants to Update the Definition for "Healthy" Claims on Food Labels A new Food and Drug Administration proposed rule, "Food Labeling: Nutrient Content Claims; Definition of Term Healthy," released on September 28, would offer new guidance to brands who label their products as "healthy" options. The FDA is looking to regulate the use of the word because of the rise of diet-related illnesses in the U.S.





























Post a Comment for "40 fda approved health claims on food labels"